Top tips:
- HCC is the sixth most common cancer, the third leading cause of cancer-related deaths, and the second most lethal cancer. It represents 90% of primary liver cancers and occurs in the presence of underlying cirrhosis most of the time.
- Patients with chronic HBV infection and patients with NAFLD/MAFLD can develop HCC in the absence of cirrhosis
- Patients with cirrhosis (Child Pugh A or B or those patients with Child Pugh C awaiting liver transplantation) and patients without cirrhosis with chronic HBV infection at increased risk of HCC should undergo HCC surveillance with ultrasound and AFP every six months.
- The diagnosis of HCC in patients with cirrhosis is based on dynamic imaging (e.g., CT, MRI or CEUS) according to well-defined criteria. Liver biopsy is needed to confirm the diagnosis in cases with atypical imaging features and in all patients without underlying cirrhosis.
- Staging and treatment allocation are based on the Barcelona Clinic Liver Cancer (BCLC) staging system, which takes into account the tumor burden, the underlying liver function, and the patient’s performance status.
- All patients with HCC must be reviewed at a Multi-Disciplinary Team (MDT) meeting before selecting therapy. This approach has increased survival rates in other series. Within Alberta, this occurs in Edmonton and Calgary.
General Cirrhosis Admission and Discharge Order Sets
*Add specific panels to general admission orders as appropriate*
For adults with cirrhosis requiring hospital admission
Cirrhosis Adult Admission Orders
For adults with cirrhosis requiring hospital discharge
Cirrhosis Adult Discharge Orders



Thank you Dr. Burak, Dr. Fung, and Dr. Moctezuma Velazquez for your efforts in creating content for this page. Check out the bottom of the page for short videos from Dr. Fung and Dr. Burak!
Risk factors and prevention
- The most important risk factor for HCC is liver cirrhosis. Hence, to prevent progression to cirrhosis, it is very important to detect chronic liver diseases in the general population early on in their natural history. Other risk factors for HCC are age, male sex, diabetes, obesity, alcohol, tobacco, and aflatoxin B1 exposure.
- Primary prevention should aim to prevent chronic liver disease by promoting a healthy diet, exercise, responsible drinking, and HBV vaccination.
- Secondary prevention should target the treatment of the etiology of the underlying liver disease, and other risk factors associated with HCC, in order to prevent disease progression and development of HCC. Coffee drinking may protect against HCC.
Note: Tertiary prevention would aim to reduce the risk of recurrence after curative treatments, but so far, there are no neoadjuvant or adjuvant therapies approved for this purpose.
Surveillance
Who should be screened:
- Patients with cirrhosis (regardless of the etiology) who are Child Pugh A or B.
- Given limited treatment options, surveillance should only be done in Child Pugh C patients if they are eligible for liver transplantation
- Patients with HBV infection without cirrhosis belonging to one of the following groups:
- PAGE-B score ≥10
- Asian females >50 years
- Asian males >40 years
- Family history of HCC
- Patients of African descent >20 years
Note: Patients with HCV-associated cirrhosis should continue to undergo screening even after SVR.
How to screen:
- Ultrasound surveillance with serum alpha-fetoprotein (AFP) every six months. Overall sensitivity of the ultrasound is 84%, but only 45% for early stages; the addition of AFP increases sensitivity to 63% in early stages.
- Do not use AFP alone for surveillance
- For ultrasound/AFP to be cost-effective, the adherence needs to be ≥34%, and the annual incidence of HCC should be ≥1.5% and ≥0.3% in patients with and without cirrhosis, respectively. Automated radiology recall programs are preferred to increase compliance – for Alberta, please see details for these programs in the grey bar.
- If there is a mass/nodule on the ultrasound > 1cm, patients need to undergo a dynamic imaging study (see diagnosis below).
- If there is a lesion < 1 cm, the ultrasound should be repeated at shorter intervals (e.g. every 3-4 months). If the lesion remains stable after 12 months of follow-up, the patient can return to every 6 months for surveillance. On the contrary, if the lesion grows or changes in pattern, the patient will need to undergo a dynamic imaging study (see diagnosis below).
NOTE: Ultrasound may perform sub-optimally in patients with obesity, steatosis, and/or multinodular livers. The US LI-RADS visualization score (i.e. A for minimal limitations, B for moderate limitations, and C for severe limitations) can help identify the need for an alternative screening strategy in a given patient (e.g. cross-sectional imaging).
Diagnosis
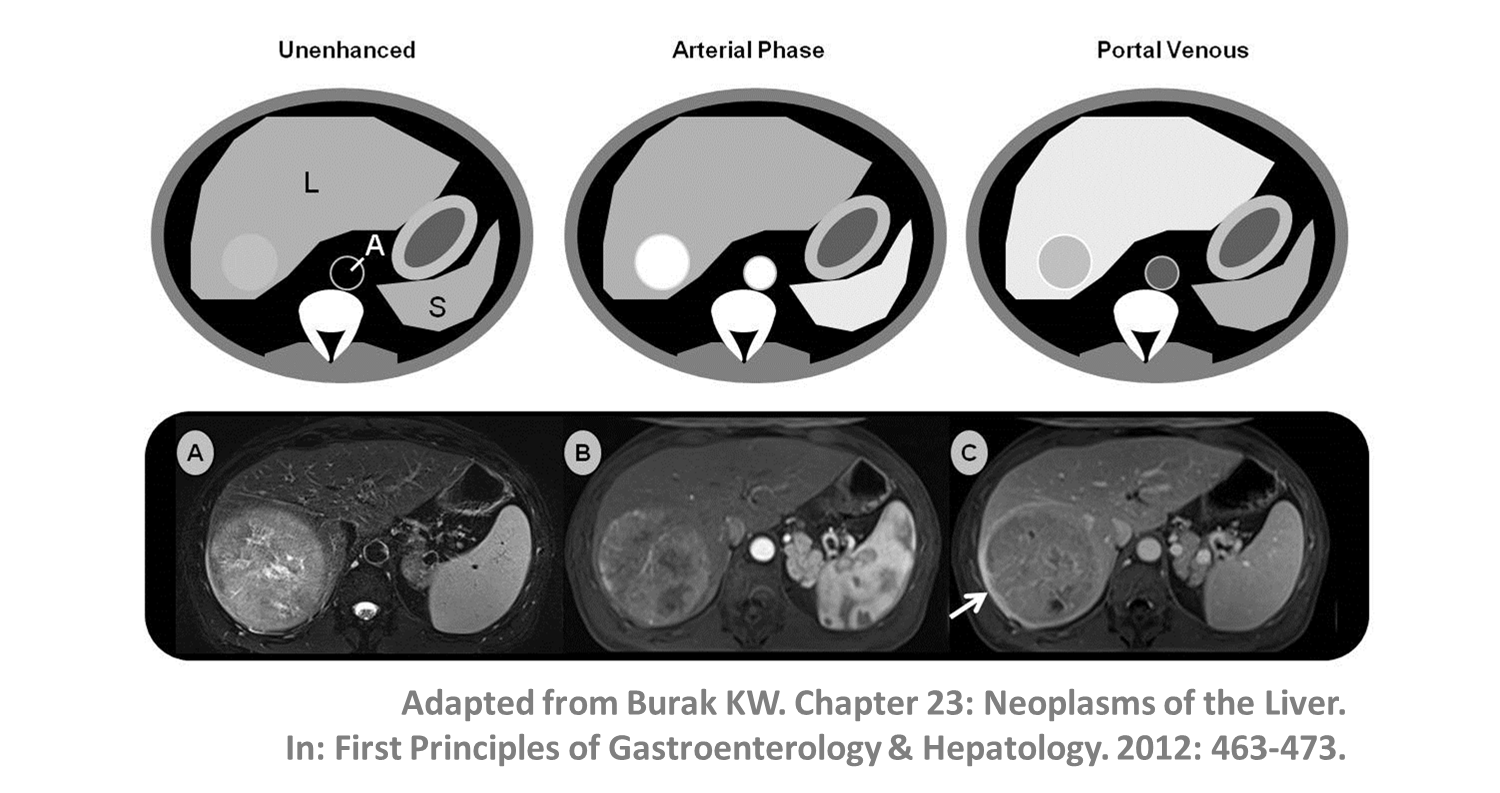
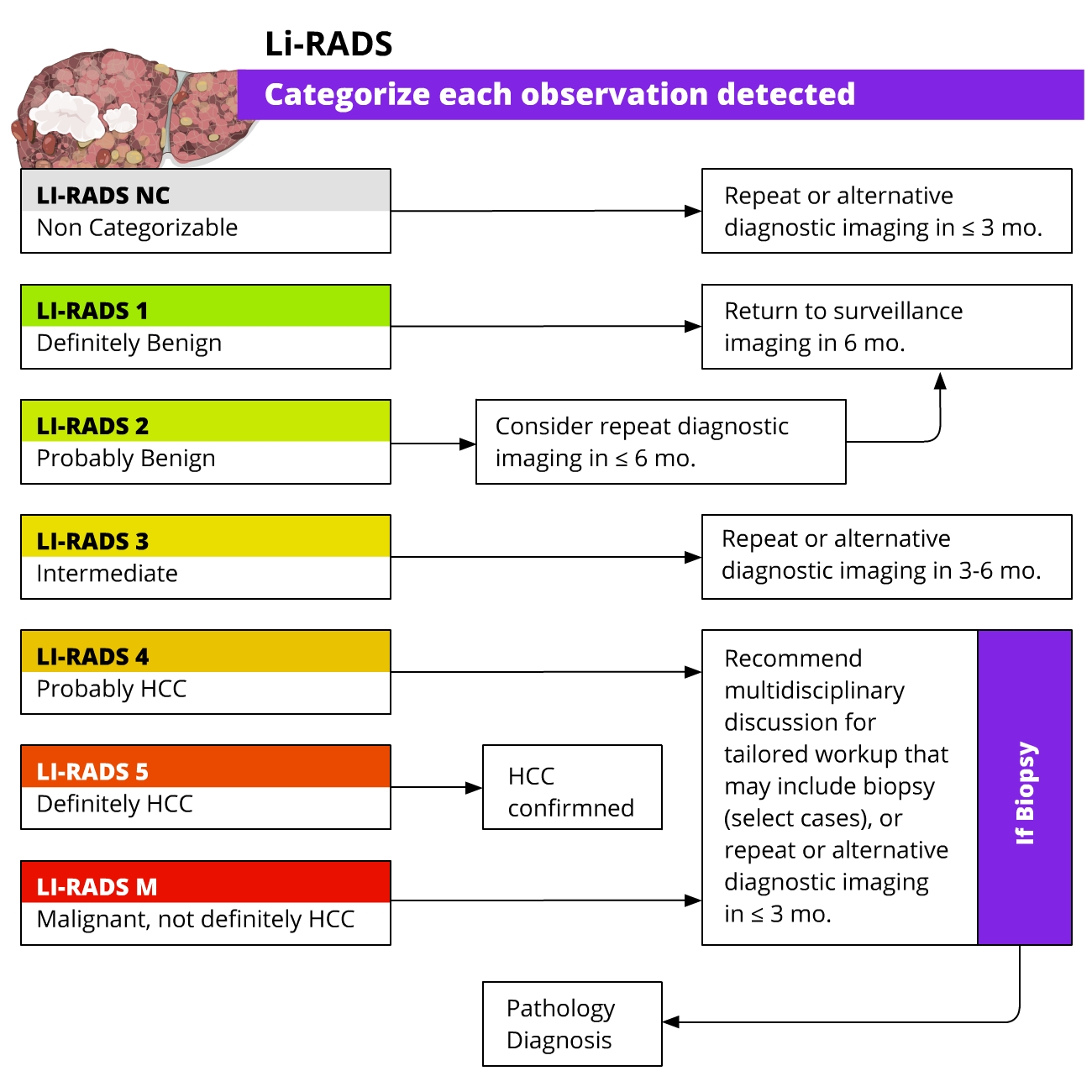
HCC- In patients with underlying cirrhosis, imaging criteria defined by the European Association for the Study of the Liver (EASL) and Liver Imaging Reporting and Data System (LI-RADS) enable HCC diagnosis (without the need for targeted biopsy).
- A lesion >1cm showing arterial phase hyperenhancement and non-peripheral venous washout can be diagnosed as HCC; LI-RADS criteria also take into account threshold growth (>50% diameter increase in <6 months).
- In patients with cirrhosis and non-conclusive imaging findings, a multidisciplinary discussion is suggested to decide the best strategy to follow: targeted biopsy, close follow-up, or repeated imaging with an alternative method.
- Liver biopsy is mandatory in non-cirrhotic patients given the lower pre-test probability of HCC.
- If a biopsy is done, specific stains may help with the diagnosis of HCC.
- Isolated alpha-fetoprotein (AFP) elevation is not required for the diagnosis of HCC (40% of HCC do not make AFP)
- AFP should not be used on its own to diagnose HCC (mixed tumors can also produce AFP).
- AFP should be ordered if HCC is suspected as it has prognostic properties and is used to define liver transplant and systemic therapy (e.g. ramucirumab) candidacy.
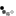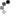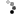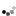
Staging and Treatment – the Alberta HCC Algorithm
-
- Once the diagnosis of HCC is confirmed, a CT of the chest should be obtained for starting.
- If there is any suspicion of bone metastasis (e.g. bone pain), a bone scan should also be obtained.
- Staging and treatment allocation are based on the BCLC staging system, which takes into account the tumor burden (e.g. number, size, vascular invasion, extrahepatic spread), the underlying liver function, and the patient´s performance status (PS).
- The BCLC comprises 5 stages:
Defined as a single lesion <2 cm in a patient with preserved liver function, without vascular invasion, extrahepatic spread, or cancer related symptoms (PS=0). The most recommended treatment for these patients is ablation, but resection is another option. Ablation and resection offer similar survival rates. These patients are not granted exception points for liver transplantation. Other treatment options, when ablation and resection are not possible, are TACE, TARE, and SBRT.
Defined as a single lesion >2cm, or a multifocal HCC with up to 3 nodules, each < 3 cm. In addition, patients need to have preserved liver function and should be free of vascular invasion, extrahepatic spread and/or cancer related symptoms (PS=0). The preferred treatment for single lesions, if possible, is resection. Liver transplant is the treatment of choice for patients with multifocal HCC or with solitary HCC < 5 cm not amenable to resection; patients that are not eligible for liver transplant should undergo ablation. If a patient with HCC is expected to be on the waiting list for > 6 months, the HCC should receive treatment as a bridging strategy to liver transplant to prevent HCC progression beyond transplant criteria. There is evidence to support the use of ablation, TACE, or TARE, or SBRT as a bridge to liver transplant. Single lesions < 8 cm can also be treated with TARE. Another alternative, when the previous strategies are not feasible, is the use of SBRT.
Refers to patients with preserved liver function, without vascular invasion, extrahepatic spread, or cancer related symptoms (PS=0), but with a tumor burden beyond that of stage A. This is a very heterogeneous stage, and can be subdivided in 3 groups:
1.Patients with a tumor burden beyond transplant criteria, but with a well defined disease that is within criteria to allow downstaging strategies. These criteria are defined by each center or transplant program. In Alberta, a patient can be considered for down-staging IF the TTV is ≤ 250 cm³, regardless of AFP. Following downstaging, a patient can be considered for transplantation if TTV ≤ 115 cm³ and AFP ≤400 ug/mL for 6 months. Downstaging can be achieved with ablation, TACE, or TARE.
2. Patients with a well defined tumor burden that is amenable to TACE, and not considered for LT. Another treatment option is TARE, which is better suited for single lesions. SBRT can also be considered.
3.Patients with diffuse, infiltrative, and/or extensive tumor burden not suitable for TACE. These patients are better served with systemic therapy.
Defined by vascular invasion and/or extrahepatic spread and/or minor cancer related symptoms (PS=1-2). These patients should be treated with systemic therapy.
Comprises patients with compromised liver function not being considered for LT, and/or patients with major cancer-related symptoms(PS>2). These patients should receive best supportive care.
Reproduced with permission from Dr. K. Burak. Source: HepAPPtology
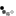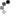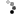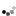
| Factor | 1 point | 2 points | 3 points |
|---|---|---|---|
| Total Bilirubin (μmol/L) | <34 | 34-50 | >50 |
| Serum Albumin (g/L) | >35 | 28-35 | <28 |
| PT INR | <1.7 | 1.71-2.30 | >2.30 |
| Ascites | None | Controlled, on diuretics | Refractory |
| Hepatic Encephalopathy | None | Controlled, on medical therapy | Refractory |
| PS | Description |
|---|---|
| 0 | Fully active and able to carry on without restrictions |
| 1 | Unable to carry out physically strenuous activities but ambulatory and able to complete work of a light or sedentary nature |
| 2 | Ambulatory and capable of all self-care but unable to complete work activities. Up and about more than 50% of waking hours |
| 3 | Capable of only limited self-care and/or confined to a bed or chair for more than 50% of waking hours. |
| 4 | Completely disabled. Unable to carry out any self-care. Totally confined to a bed or chair. |
- Must have preserved liver function (Child Pugh A) with no significant portal hypertension (no varices, hepatic venous pressure gradient or HVPG < 10 mmHg, platelets >100 or the combination of LSM-TE < 15 kPa and platelets > 150) and a normal bilirubin to avoid post-op liver failure
- Recurrence occurs in 70% at 5 years
- RCTs vs RFA show similar survival but fewer recurrences after surgery in the early stage
- Complications include post-op infections and liver failure (2% mortality)
- Laparoscopic resection has a lower rate of postoperative complications and can be considered in selected cases even in patients with clinically significant portal hypertension
- Lesions should be <3cm and there should be < 3 lesions
- Can be performed in Child Pugh A or B (those who are not candidates for surgery)
- Meta-analyses of RCTs show that RFA is better than PEI for local control & disease free survival if tumour >2cm but PEI is preferred if concerns about thermal injury to adjacent structures or proximity to blood vessels where heat-sink can be an issue with RFA
- Compared to RFA, MWA achieves more tumor necrosis and is less prone to heat sink effect
- Recurrence also 70% at 5yrs (>2.5 cm predicts recurrence)
- Complications in 2-5%: bleeding, thermal injury, abscess, tumour seeding (1%)
- LT is the only therapy that treats both the HCC and underlying cirrhosis, so it can be done in Child Pugh C
- Milan criteria [single <5cm, 3 HCC <3cm] predicts low risk for recurrence (<15%)
- Patient require long-term immunosuppression, which has many complications, and organ donor shortage lead to many patients progressing beyond accepted criteria while waiting for LT
- Takes advantage of the rich arterial blood supply of tumours to provide chemotherapy (usually doxorubicin) with embolic particles (induces tumour ischemia)
- Need to have good liver function (CPA or CPB7) to prevent liver failure from ischemia, and must avoid if there is a main portal vein (PV) thrombosis (chance of liver failure)
- Caution if bilirubin >34 umol/L or ascites especially if not a candidate for transplantation
- Consideration should be given to systemic therapy for tumor burden beyond up to seven criteria, because these tumors may be associated with worse outcomes after TACE.
- Use of drug eluting beads (DEB-TACE) provides a more standardized technique and is better tolerated (but more expensive), but offers no survival advantage
- Complications include post embolization syndrome (fever, nausea, vomiting) in approximately 25%, ischemic complications and rarely liver failure (2-3% mortality)
- Like TACE it should be reserved for CPA or CPB7
- Yttrium (Y90) microspheres provide internal radiation to the tumour
- Particles are smaller and less embolic and can be used if PV thrombosis
- Cohort studies and small RCTs show longer time to progression when compared to TACE, but with no survival advantage
- May be better than TACE for large tumours and for bridging patients to LT
- It is more expensive than TACE and requires two angiograms (first to calculate shunt fraction to lungs and to embolize collaterals) but it can be done as an outpatient as post embolization syndrome is uncommon.
- Better results with personalized dosimetry and when targeting single lesions
- Complications include radiation damage to liver, lungs or GI tract. TARE Referral form
- Because there is a lack of robust evidence, SBRT is usually reserved for patients who aren’t candidates for other locoregional therapies
- Provides confocal external radiation in 5 fractions
- Cohort studies show good local control
- Complications include radiation damage to liver
- Other SBRT considerations from the Cancer Institutes in Edmonton and Calgary:
- Good performance status (ECOG 0 -2)
- Life expectancy >6 months
- Limited number of metastases (<3-5)
- Limited prior overlap RT
- Patient able to tolerate RT position
- Limited tumor size (< 6 cm)
- Controlled or absent extra hepatic disease
- At least 700 cc of uninvolved liver volume
- Adequate liver function (Child Pugh A)
- Adequate hematologic function (Absolute neutrophil count (ANC) ≥ 1,500 cells/mm3, Platelets ≥ 70,000 cells/mm3, Hemoglobin ≥ 8.0 g/dl)
- Additional contraindications to radiotherapy
- Tumor location close to radiosensitive structures like the stomach, small bowel, large bowel, kidney
- Ascites - poor reproducibility and represents poor liver reserve
Systemic therapy
- Combination of immunotherapy and targeted therapy
- Atezolizumab (monoclonal antibody checkpoint inhibitor that blocks PD-L1) and bevacizumab (monoclonal antibody that blocks VEGF) showed statistically improved overall survival
- When compared to sorafenib [19.2 vs 13.4 months, HR 0.66 (95% CI: 0.52, 0.85)], and also a longer time to deterioration of quality of life (11.2 vs 3.6 months).
- First line systemic therapy of choice
- Patients need to have a recent upper endoscopy and if varices are present, adequate prophylaxis should be in place before first starting treatment.
- Compared to sorafenib, patients have more hypertension and proteinuria, but less diarrhea and hand-foot skin reaction.
- Contraindicated in liver transplant recipients
- Caution in patients with autoimmune conditions
- Targeted oral therapy with anti-VEGF (vascular endothelial growth factor), PDGF (platelet derived growth factor), RAF kinase activity
- Can be used as a first line systemic therapy when there is a contraindication for atezolizumab-bevacizumab
- RCTs have shown a survival benefit over placebo in patients with preserved liver function (Child Pugh A)
- Side-effects include nausea, diarrhea, hand & foot skin reactions and hypertension
- There is real-world evidence to support its use in Child-Pugh B patients
- Can be used after liver transplant
- Targeted oral therapy that blocks VEGF receptors, FGF (fibroblast growth factor) receptors, PDGF receptor, RET, and KIT
- Found to be non-inferior to sorafenib in large open-label phase 3 study (median
- Survival 14 months for levatinib vs 12 months for sorafenib) with higher tumour response rates. Trial excluded patients with main portal vein invasion and/or bulky tumors (>50% of liver parenchyma involved).
- Can be used as a first line systemic therapy when there is a contraindication for atezolizumab-bevacizumab
- Side-effect profile similar to sorafenib
- Targeted oral therapy against FGFR, VEGFR, RAF, TIE2, PDGFR
- Approved as a second line therapy after progression with sorafenib, it improved survival compared to placebo [10.6 vs 7.8 months, HR 0.63 (0.50-0.79)].
- Patients need to have tolerated sorafenib in order to be eligible for this therapy
- Side-effect profile similar to sorafenib
- Targeted oral therapy against VEGFR and MET
- Can be used as a second or third line agent. A RCT showed improved survival compared to placebo in patients that had progressed after at least one prior systemic therapy
- Side-effect profile similar to sorafenib
.
- Monoclonal antibody against VEGFR2
- Improved survival compared to placebo as a second line agent (after sorafenib) in patients with AFP> 400 ng/ml
- Compared to sorafenib, ramucirumab was associated with more headache, hypertension, proteinuria, and fluid retention (leg edema and ascites), but less diarrhea and hand-foot skin reaction.
Palliative care considerations for HCC
- Palliative care principles should be followed in all patients with HCC – this basic/primary palliative care can be carried out by any physician involved in the patient’s circle of care (Primary care physician, Oncologist, Hepatologist) and at a minimum includes advance care planning and goals of care discussions.
- Specialist palliative care involvement (a board certified specialist or the primary care physician if they are comfortable with palliative care) should be considered with complex palliative care including refractory symptom burden or hospice referral that is not straight-forward.
Prognosis
Can be defined based on the stage:
- Very early and early: Median survival of more than 5 years
- Intermediate: Median survival of more than 2.5 years (16 months without treatment)
- Advanced: Median survival of more than 2 years. (7 months without treatment)
- Terminal: Median survival of 3 months.
Introducing Dr. Fung and Dr. Burak
Patient materials:
You can direct patients to the following:
What is HCC
Downloadable content:
You can download these to print or view offline:
AHS Hepatocellular Carcinoma Clinical Practice Guideline Version 8
AASLD Staging, Guidance, and Management Guidelines
AASLD HCC Treatment Guidelines
EASL Clinical Practice Guidelines:
References:
This section was adapted from content using the following evidence based resources in combination with expert consensus. The presented information is not intended to replace the independent medical or professional judgment of physicians or other health care providers in the context of individual clinical circumstances to determine a patient’s care.
Authors: Dr. Kelly Burak, Dr. Chris Fung, Dr. Aldo Montano Loza, Dr. Carlos Moctezuma Velazquez, Dr. Puneeta Tandon
References:
- EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018 Jul;69(1):182-236 PMID 29628281
- Heimbach JK et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018 Jan;67(1):358-380 PMID 28130846
- Sherman M, Burak K et al. Multidisciplinary Canadian consensus recommendations for the management and treatment of hepatocellular carcinoma. Curr Oncol 2011; 18(5):228-240. PMID 21980250
- Burak KW, Kneteman NM. An Evidence-Based Multidisciplinary Approach to the Management of Hepatocellular Carcinoma (HCC): The Alberta HCC Algorithm. Can J Gastroenterol 2010; 24(11): 643-650. PMID 21157578.
- Reig M, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol, 2022. PMID 34801630
- Bruix J, Systemic treatment of hepatocellular carcinoma: An EASL position papeJ Hepatol 2021, 75(4):960-74. PMID 34256065
Last reviewed December 14, 2022.